A dangerous element
Tracking the elusive biogeochemistry of mercury
Along Northern California’s coastline, scientists have found concerning levels of mercury in two top predators. In the Santa Cruz Mountains, Peter Weiss-Penzias, a UC Santa Cruz associate researcher in microbiology and environmental toxicology, discovered that coastal-dwelling pumas have three times the amount of the toxic heavy metal in their fur and whiskers compared to inland mountain lions. And in UCSC dining halls, ecotoxicologist Myra Finkelstein, an adjunct associate professor, documented that some students were eating more than 20 servings of tuna a week—enough to accumulate potentially harmful levels of mercury in their bodies.
Both cases are worrisome. Mercury is a neurotoxin, and high enough doses degrade the mental capacities and motor skills of those exposed. In pregnant mothers, the metal can enter the womb and harm infant brains. And the silvery element can build up in the living tissues of both plants and animals, compounding the danger.
Despite relatively recent laws and treaties that now limit its release, a lot of mercury has wound up in bodies of water—creeks, rivers, lakes, and the ocean—where it transforms into its most toxic form and enters food chains. In addition, key steps in the metal’s movements through the environment—its “biogeochemistry”—remain unclear, including how it changes from its less dangerous, inert forms to an organic molecule that accumulates in living tissue. To better understand the mercury threat, UCSC scientists are working to learn how this mysterious element shifts between its different forms. “Nothing is simple when it comes to mercury,” said Weiss-Penzias. “It has a way of disappearing and then reappearing somewhere else.”
Gold and coal
The elevated levels of mercury in the environment today are the result of its current and historic use in gold mining, and from the continued use of coal for energy production. Gold Rush–era mining contaminated watersheds throughout California. Miners used the shiny liquid quicksilver—mercury in its pure, elemental form—to recover gold from gravel deposits adjacent to rivers. At these placer mines, miners washed gold-containing sediments through sluices lined with quicksilver. The mercury and gold formed a heavy amalgam easily sifted from other sediments. In the process, much of the metal escaped and drained into rivers and streams. An estimated 10 million pounds of mercury entered the environment from placer mining throughout California. The San Francisco Bay is tainted by this past, as are the 131 reservoirs identified by the state as “mercury-impaired.”

Today, small-scale gold mining in Asia, Africa, and South America still relies on mercury—and may account for over half of ongoing global mercury pollution. The remainder comes primarily from coal-powered energy production. Millions of years ago, the metal glommed onto the ancient plant and animal remains that eventually became coal. Now, burning this fossil fuel releases mercury into the atmosphere as a gas that drifts around the globe.

Thanks to gold mining and coal burning, a lot of mercury contaminates the environment. “The general consensus is that we’ve increased the amount of mercury moving around the active reservoirs of the Earth—the ocean, soils, and the atmosphere—by somewhere between a factor of three and seven,” said associate professor of ocean sciences Carl Lamborg, an expert in the biogeochemistry of mercury. “Seventy-five percent or more of all the mercury in the air around you right now is a consequence of human activity.”
Mobilized metal
All this mobilized mercury exists in three main forms. Its elemental form, Hg0, the liquid quicksilver at room temperature, easily volatilizes into a gas. That gaseous mercury becomes charged in the atmosphere to its Hg2+ form, or mercuric ions. These ions can diffuse into water. Under low-oxygen conditions, such as in lake bottom, coastal, and seafloor sediments, bacteria convert mercuric ions into methylmercury (CH3Hg+ ions. Toxicologists worry especially about methylmercury. “The mercury in our bodies is mostly methylmercury,” said Lamborg. “Methylmercury by itself is not necessarily more toxic than any other form of mercury, but it sits there as a reservoir that bleeds out into the rest of your tissues.”
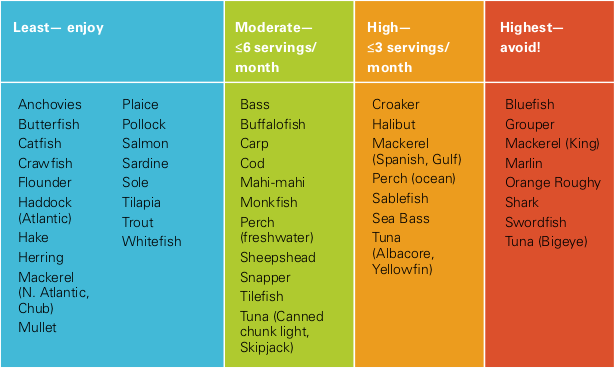
That methylmercury comes mostly from eating fish, especially top predator fish. In the ocean, methylmercury sticks to phytoplankton, which are eaten by small fish, which are eaten by tuna, swordfish, and sharks. At every step up the food chain, the metal becomes more concentrated. Each tiny plankton accumulates mercury across its life, and fish eat these concentrated morsels. Plankton eaters have about 100 times more mercury in their bodies than what’s in their food. And top predators—like the tuna served at UCSC dining halls—have mercury concentrations another 100 times higher than plankton eaters. When we eat tuna, more than 90 percent of the methylmercury in the fish remains in our bodies, stored mostly in fat. From there, some slowly releases as mercuric ions, which is what does the damage to our tissues. To play it safe, Finkelstein recommends minimizing your exposure by being careful about what type and how much fish you eat (see table below).
But fish is apparently not the only way mercury gets into the bodies of land animals. In 2010, while working in the Elkhorn Slough, an estuary on the coast about halfway between Santa Cruz and Monterey, Weiss-Penzias wondered about the thick fog that rolled in every day. When he analyzed fog droplets for mercury, the concentration was much higher than he expected. In research that followed, Weiss-Penzias showed how this mercury entered the food chain: lichen and plants absorbed the fog, deer eat the lichen and plants, and mountain lions eat the deer. One coastal mountain lion he sampled (via its fur and whiskers) had levels of mercury known to be toxic to mink and otters, and two others showed concentrations that could cause confusion and potentially impair their ability to breed and parent kittens.
Toxic pathways
While the mercury in tuna, humans, and pumas ultimately traces back to the methylmercury generated in the ocean by anaerobic bacteria, exactly how these microbes transform elemental mercury into methylmercury remains unknown. It’s not a result of their metabolism—these anaerobes eat organic matter and breathe sulfate or oxidized iron. Another process must drive the change.
And although these microbes typically live in low-oxygen environments, mercury methylation has been shown to occur even in well-aerated ocean waters that contain few methylating bacteria. This suggests there’s probably another pathway to methylmercury in addition to the one associated with the anaerobes. According to Feiyue Wang, professor of biogeochemistry at the University of Manitoba in Canada, there’s a “bizarre” shallow layer of methylmercury in the Arctic Ocean, even though those waters are well-oxygenated. “In that kind of water, we normally would not anticipate a large population of anaerobic bacteria,” Wang said. “This newly discovered phenomenon really forces us to consider other possibilities.”
To investigate the mercury methylation process, Lamborg has directed research to selectively filter seawater to narrow down the possibilities by a process of elimination. In an experiment performed by graduate student Kathleen Munson, now a postdoctoral fellow at the University of Manitoba, methylmercury continued to form in seawater samples after all the microbes were filtered out. Lamborg has some leads on the mystery reaction. Bacteria produce vitamin B12, and that could react with mercuric ions to produce methylmercury. “There's all this circumstantial evidence suggesting that we should be looking for a B12-related mechanism,” he said.
Another enigma lies at the bottom of the San Francisco Bay, where the sediments contain an estimated five-fold increase in mercury from natural levels due to gold production activity, including from mercury mining (see sidebar). However, the fish reeled in contain low levels of mercury. One explanation is that most of the mercury remains in the inert form in which it was mined, mercury sulfide (known as cinnabar, with its characteristic red color). “It should make us all nervous,” said Lamborg. “If we messed with that system in some way, that could make that mercury suddenly bioavailable to methylating bacteria.”

Living with it
A better understanding of mercury’s elusive movements could help to minimize its toxic effects. It could, for instance, suggest ways for clean-up efforts at contaminated sites to keep the sedimentary mercury in its inert cinnabar form. But even with targeted clean-up efforts at some reservoirs and wetlands, we can’t do anything about most of the mercury we’ve already unleashed. “You can’t clean up the ocean, you can’t clean up the atmosphere,” said Weiss-Penzias. “It’s just too dispersed.”
Meanwhile, Weiss-Penzias continues to study how mercury makes its way into the mouths of humans and other animals. After he published his study on the element in pumas, he received many concerned inquiries, including from a farmer who worried about their goats eating lichen. Weiss-Penzias hopes an experiment he’s planning can help address some of the concerns, by measuring how mercury-laden fog might be affecting strawberries, broccoli, and artichokes grown on the Central Coast.
More education about mercury exposure is probably also a good idea. In her dining hall study, Finkelstein found that nearly all the students were unaware of the risk associated with eating too much tuna. “Many students seem to have limited to no understanding,” she said. “It was alarming.” Finkelstein said that while a national educational effort has aimed to keep women from eating mercury-rich fish during pregnancy, the risk to the general public—including to children and young adults, whose developing brains may also be vulnerable—isn’t widely acknowledged. ”It has changed what I allow my children to eat,” Finkelstein said. “I think about it a lot. We are exposed to such a large amount of chemicals on a daily basis.”
This risk—and the element’s enigmatic ways—keep the UCSC mercury scientists engaged. “I’ve been working in the field for almost 20 years trying to understand the fate and transport of mercury in the environment,” said Weiss-Penzias. “It’s still quite vexing.”